Ensure regulatory compliance, protect intellectual property, and streamline operations—all within a centralized platform. FacilityOS empowers pharmaceutical facilities to improve operational efficiency, enhance security, and maintain audit-ready records with ease.
Facility Management Platform for Pharmaceutical Industry
A Secure, Compliance-Ready Facility Management Solution for Pharmaceuticals

Experience the Smarter Way to Run Your Pharmaceutical Facility
Pharmaceutical facilities operate under some of the strictest compliance and security standards, with oversight from regulatory bodies like the FDA and DEA. In this highly regulated environment and increased security and safety standards, pharmaceutical companies need to uphold the highest safety and efficiency standards.
Enterprise facility, asset and visitor management platform, FacilityOS, gives you the tools to digitize and streamline operations and processes while effortlessly enforcing safety protocols, driving site security requirements, and achieving regulatory compliance.
Compliance and Reporting
FacilityOS simplifies regulatory adherence by capturing digital records and audit trails eliminating manual processes while supporting FDA, DEA, and ISO 13485 requirements.
Centralized Data Across Multiple Sites
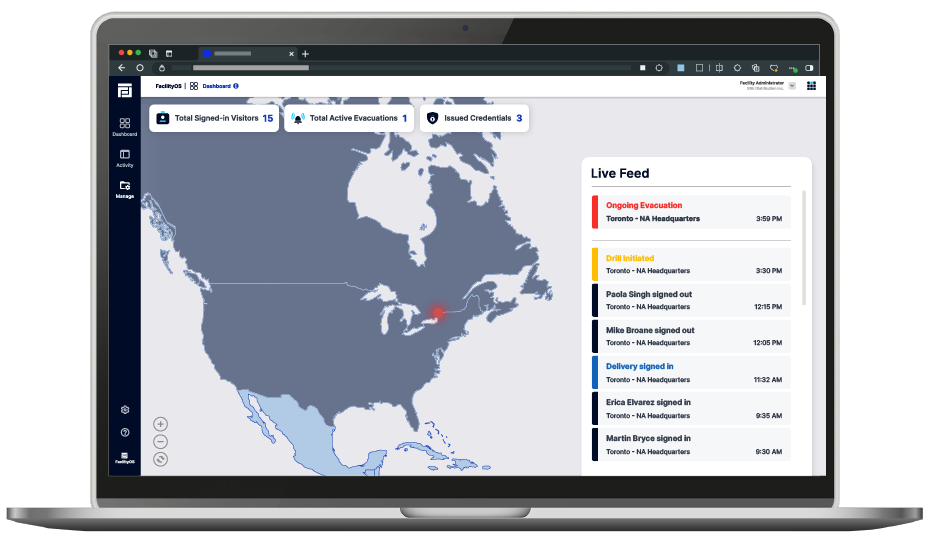
FacilityOS centralizes data in a single, easy-to-use dashboard, giving you complete visibility providing instant access to visitor logs, emergency management data, physical access credentials issued and more. This centralized system reduces administrative overhead and helps ensure consistent reporting across your organization.
Security and Privacy
From maintaining robust visitor screening protocols to streamlining mailroom management, central receiving, and asset logistics workflows, FacilityOS offers enhanced security for pharmaceutical companies. Features like watchlists, access restrictions, and encrypted data storage help you manage risks and protect sensitive information while ensuring all interactions are logged and auditable.







FacilityOS Platform for the Pharmaceutical Industry
FacilityOS is a comprehensive modular platform designed to help pharmaceutical facilities streamline operations, driving efficiency across your facility. Whether you need a complete solution or just a visitor management system, FacilityOS scales to meet your needs, delivering measurable results that support compliance and reduce operational disruptions.
Why Do Pharmaceutical Companies Needs a Facility Management System?
Optimize for Simplified Compliance
Flexible Modular Platform
FacilityOS is a configurable platform designed to meet the unique needs of manufacturing facilities. With modules for visitor management, emergency management, contractor compliance management, logistics management, and physical identity and access management, the platform streamlines and automates processes that mitigate risk and proactively help pharmaceutical companies enforce safety protocols.

One Platform, Complete Oversight
Centralized Records for Compliance
All critical data, from visitor logs and evacuation details to contractor and logistics information, are centralized in the FacilityOS platform providing a comprehensive audit trail. This unified system supports compliance and strengthens security protocols, allowing you to make quick, informed decisions while reducing reliance on manual records and minimizing errors.

Seamless Growth, Maximum Flexibility
Built to Evolve with Your Needs
FacilityOS is designed to scale and grow with you. As your operations expand, you can seamlessly incorporate complementary modules or extend functionality across multiple facilities to meet evolving operational and regulatory needs. With an unlimited site-level license model, there’s no limit to the number of people accessing the platform, ensuring your team has the tools to maintain safety, compliance, and efficiency at every growth stage.


Intuitive, easy to use
We rolled out 90 locations quickly in about 3 months with the help of iLobby's support team.
Jeff O.
Field Service Leader


iLobby is EXCELLENT for contractor management!!
even used the iLobby system to warn us if contractors had not left the site after a certain period of time, but typically should have.
Dee M.
Health Safety Environment Coordinator


I highly recommend iLobby
Knowing who is at your facility and having the ability to alert them to evacuate if necessary is exceptionally beneficial.
Todd C.
EHS Manager


iLobby runs itself
instance viewing of everyone coming and going plus add visitors in advance.
Robert I.
IT Automation Technician


Ton of features
Solved our problem of maintaining annual paperwork for rules and regulations for visitors and contractors.
Nicole F.
Food Safety and Quality Assurance Manager


“Great Experience”
A lot more robust & easier to navigate than paper. Emergency notices are also a big feature that we use in the case of emergencies at our sites to communicate to our employees.
Travis E.
IT Plant Support Analyst, Infrastructure & Security


“Extremely Versatile”
This software allows us to notify our employees in case of emergency all with one simple set up per employee.
Jennifer A.
Manager, General Services Lactalis USA


"iLobby is EXCELLENT for contractor / visitor management!!"
The iLobby system is easy to use, the software is user friendly, and there are multiple ways to take advantage of the capabilities of the system.
Dee M.
Health Safety Environment Coordinator


"Ease of implementation and use."
Visitors have been impressed with how easy and advanced our Check-in process is.
Chad J.
Director of Supply Chain


"Definitely recommend"
We have experienced zero issues with visitors checking in which is all you can ask for with a visitor mgmt platform!
Molly V.
Executive Assistant, Operations


"Easy set up, easy to use"
Its easy to use, very customizable and was very quick and simple to implement.
Billy L.
Building Automation Systems Manager


"A very user friendly product!
We had a very easy time implementing this as our visitor management solution.
Usama M.
IT Supervisor, Plant Support

Facility Management Platform Modules
Streamline Operations Management with FacilityOS
Book a Demo