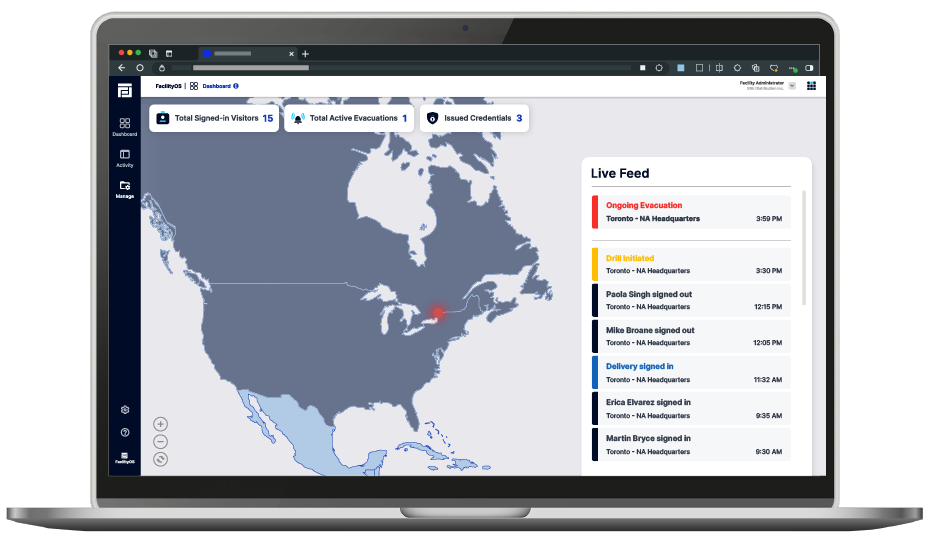
VisitorOS is a powerful visitor management system designed to address the unique needs of the pharmaceutical industry. This robust solution digitizes and automates the entire visitor journey helping pharmaceutical organizations to improve operational efficiency while helping to ensure compliance with stringent regulations.
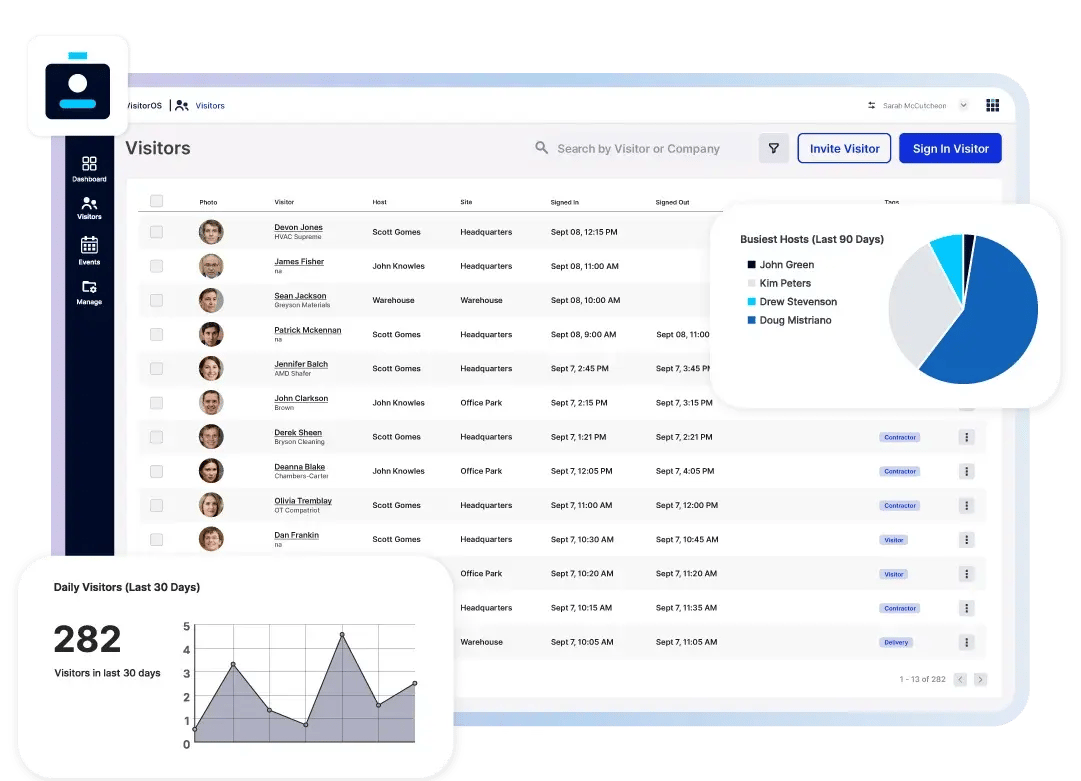
Why Pharmaceutical Organizations Need Visitor Management
The pharmaceutical industry requires high standards of security and compliance, especially when it comes to protecting intellectual property, research data, and controlling access to sensitive areas such as laboratories and manufacturing floors. VisitorOS helps organization enhance workplace safety, security and compliance by managing and providing visibility into who is in their building. From registration to sign-out, VisitorOS ensures that pharmaceutical companies maintain the highest standards of safety, security, and regulatory compliance.
How Can VisitorOS Help the Pharmaceutical Industry?

Digitize the Sign-In/Out Process
Eliminate manual check-ins with a secure, digital visitor management system. VisitorOS automates the entire sign-in and sign-out process, improving accuracy and operational efficiency. All visitor data is securely stored in a centralized dashboard, providing easy access during audits and compliance reviews.

Gain Real-Time Visibility
Stay informed with real-time visitor insights. VisitorOS offers a comprehensive view of on-site visitors and expected arrivals, empowering pharmaceutical security and management teams to monitor facilities effectively.

Comprehensive Audit Trails
VisitorOS automatically captures every interaction—from sign-in to sign-out—creating a complete audit trail. These time-stamped records ensure compliance with industry standards and facilitate readiness for inspections or internal reviews, supporting regulatory compliance and security policies.

Tailor Workflows
Customize visitor sign-in procedures, pre-registration steps, and access requirements to align with pharmaceutical industry standards. Whether you need specialized security screenings or custom documentation collection, VisitorOS adapts to your specific operational needs.

Ensure Document Compliance
Automate the distribution and tracking of critical documentation such as non-disclosure agreements (NDAs), safety briefings, or regulatory certifications. VisitorOS ensures that all necessary documents are acknowledged by visitors before access is granted, reducing risk and improving adherence to internal protocols.

Strengthen Visitor Security
Enhance visitor screening by integrating third-party watchlists and creating custom internal lists. With automated flagging and alerts, VisitorOS helps prevent unauthorized access to your facility.
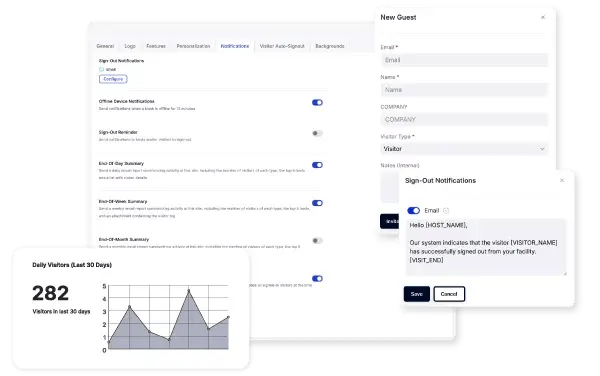
Keep Teams Informed
Ensure key personnel stay informed with real-time updates regarding visitor activity. Whether it's daily, weekly, or monthly visitor logs, device alerts, or immediate notifications of watchlist hits, our system ensures the right information reaches the right people at the right time.

Enhance Security with Badge Printing
Issue visitor badges instantly upon sign-in to ensure proper identification and compliance with facility security policies. VisitorOS badges can include photos, expiration times, and access restrictions to align with pharmaceutical industry security standards.

Streamline Event Visitor Management
Simplify visitor management for pharmaceutical events and conferences. With pre-registration, security screening, and streamlined check-in, VisitorOS ensures that events run smoothly while maintaining high security standards throughout the process.

Protect Visitor Privacy
Safeguard visitor data with industry-leading privacy controls. VisitorOS helps pharmaceutical companies comply with stringent data security regulations such as GDPR, ensuring that all personal information is encrypted, securely stored, and managed in full compliance with relevant privacy laws.
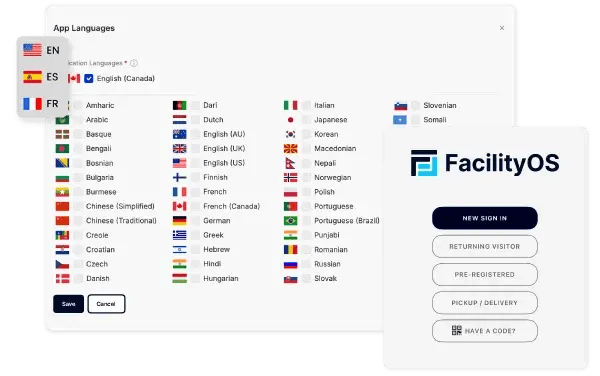
Multilingual Support
Ensure a smooth and welcoming experience for all visitors with multilingual support. VisitorOS offers multiple language options to accommodate diverse visitor needs, ensuring clear and effective communication in any language.
Pharmaceutical Industry Compliance Requirements
Pharmaceutical companies must adhere to rigorous industry regulations and standards to protect patient safety, secure sensitive data, and ensure quality manufacturing processes. VisitorOS helps streamline compliance by automating visitor data collection, maintaining audit trails, and ensuring access control policies are followed. With centralized, auditable records, pharmaceutical companies can easily pass inspections and audits without added administrative burden.
General Data Protection Regulation (GDPR)
GDPR governs how personal data of EU citizens is collected, stored, and processed. VisitorOS helps pharmaceutical companies ensure compliance with GDPR by:
- Offering tamper-proof kiosks and secure sign-in processes to prevent unauthorized access.
- Supporting data retention and deletion controls that align with GDPR requirements.

Federal Drug Administration (FDA)
The FDA requires pharmaceutical companies to maintain strict standards for electronic records and signatures. VisitorOS helps meet these requirements by:
- Offering secure, electronic sign-in procedures that create traceable records for all visitors.
- Maintaining audit-ready logs that capture every visitor interaction, supporting regulatory audits and inspections.
- Allowing customization of sign-in workflows to include necessary FDA compliance documentation.

Who Benefits from a Visitor Management System?
Security Managers
Security Managers rely on robust systems to prevent unauthorized access. VisitorOS provides real-time monitoring, automated alerts, and secure check-in processes that ensure only authorized individuals access sensitive zones, supporting industry security standards.
Facility Managers
Facility Managers in pharmaceutical facilities ensure that all visitor interactions are efficiently managed, maintaining strict regulatory compliance. VisitorOS streamlines visitor check-ins, secures sensitive areas, and keeps detailed audit trails to support adherence to GMP and FDA guidelines.
Visitors & Contractors
Pharmaceutical facilities host a diverse range of visitors, including regulatory inspectors, contractors, and suppliers. VisitorOS simplifies registration and identification, integrates with emergency protocols, and ensures that every visitor adheres to established safety and compliance standards.
EHS Managers
EHS Managers focus on maintaining a safe, compliant environment. VisitorOS aids in monitoring visitor movements, ensuring that all interactions align with OSHA, Health Canada, and ISO safety protocols, thereby enhancing overall workplace safety.

Intuitive, easy to use
We rolled out 90 locations quickly in about 3 months with the help of iLobby's support team.
Jeff O.
Field Service Leader


iLobby is EXCELLENT for contractor management!!
even used the iLobby system to warn us if contractors had not left the site after a certain period of time, but typically should have.
Dee M.
Health Safety Environment Coordinator


I highly recommend iLobby
Knowing who is at your facility and having the ability to alert them to evacuate if necessary is exceptionally beneficial.
Todd C.
EHS Manager


iLobby runs itself
instance viewing of everyone coming and going plus add visitors in advance.
Robert I.
IT Automation Technician


Ton of features
Solved our problem of maintaining annual paperwork for rules and regulations for visitors and contractors.
Nicole F.
Food Safety and Quality Assurance Manager


“Great Experience”
A lot more robust & easier to navigate than paper. Emergency notices are also a big feature that we use in the case of emergencies at our sites to communicate to our employees.
Travis E.
IT Plant Support Analyst, Infrastructure & Security


“Extremely Versatile”
This software allows us to notify our employees in case of emergency all with one simple set up per employee.
Jennifer A.
Manager, General Services Lactalis USA


"iLobby is EXCELLENT for contractor / visitor management!!"
The iLobby system is easy to use, the software is user friendly, and there are multiple ways to take advantage of the capabilities of the system.
Dee M.
Health Safety Environment Coordinator


"Ease of implementation and use."
Visitors have been impressed with how easy and advanced our Check-in process is.
Chad J.
Director of Supply Chain


"Definitely recommend"
We have experienced zero issues with visitors checking in which is all you can ask for with a visitor mgmt platform!
Molly V.
Executive Assistant, Operations


"Easy set up, easy to use"
Its easy to use, very customizable and was very quick and simple to implement.
Billy L.
Building Automation Systems Manager


"A very user friendly product!
We had a very easy time implementing this as our visitor management solution.
Usama M.
IT Supervisor, Plant Support

What Can VisitorOS Do for You?
Request a demo to see how VisitorOS can deliver immediate results for your organization.
Book a Demo
This webpage and its content are an interpretation of the compliance requirements and is not legal advice nor should it act as a replacement for having a legal team review the specific compliance needs of your organization.