Logistics & Asset Management Platform
Secure Pharmaceutical Supply Chain Logistics
Why Pharmaceutical Companies Need Logistics Management
The pharmaceutical supply chain is complex—requiring strict regulatory adherence, precise inventory control, and traceability from manufacturing to distribution. Without an advanced logistics system, inefficiencies, temperature excursions, and supply disruptions can put patient safety and compliance at risk. LogisticsOS centralizes pharmaceutical logistics management, ensuring full traceability, regulatory compliance, and enhanced operational efficiency through automated workflows and secure chain-of-custody tracking.
How Can LogisticsOS Help Pharmaceutical Supply Chains?

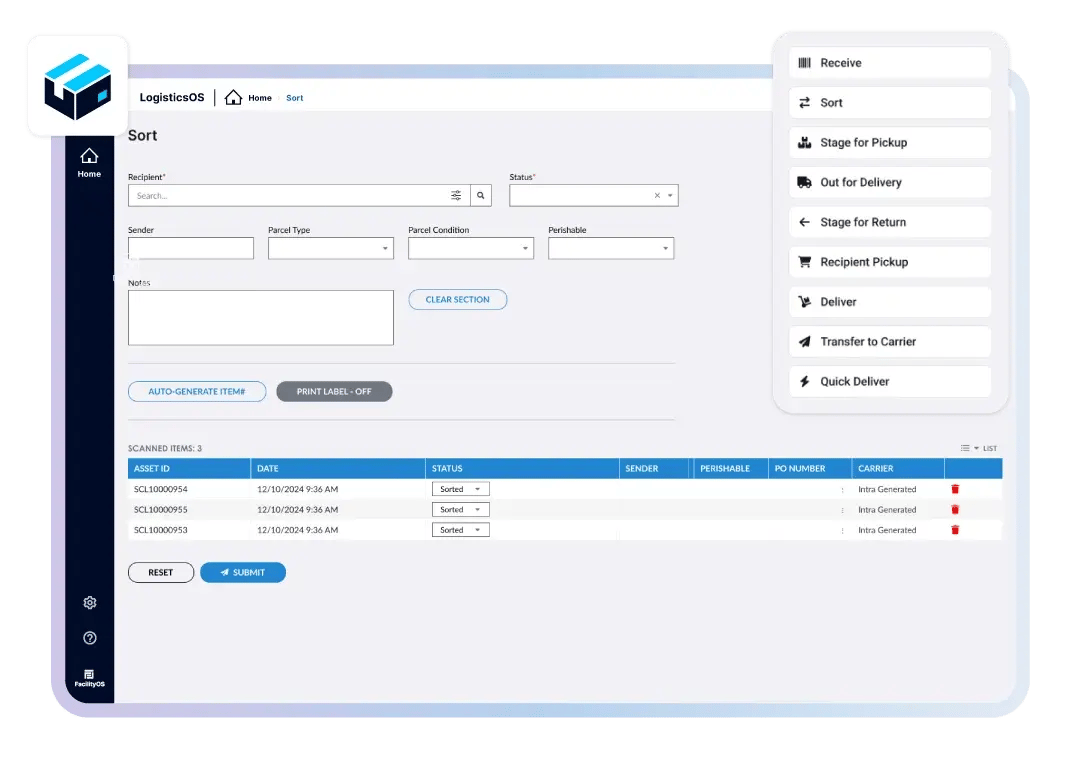
Streamline Chain of Custody Management
Digitize chain-of-custody processes to eliminate manual risks. With automated updates and detailed tracking, LogisticsOS ensures secure, error-free, and searchable transaction records for every pharmaceutical product, from raw materials to patient-ready medications.
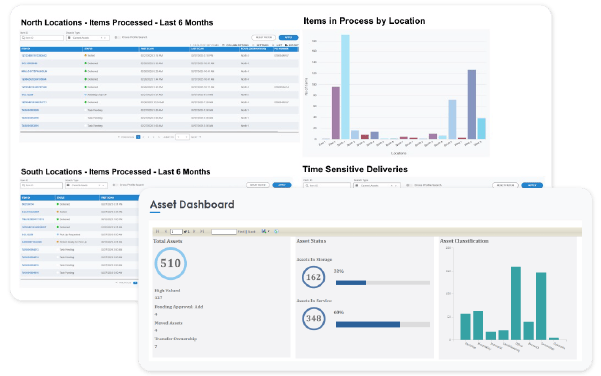
Gain Actionable Insights with Comprehensive Reporting
Leverage real-time dashboards and analytics to optimize pharmaceutical logistics. Performance metrics help identify inefficiencies, track batch movements, and improve cold-chain and compliance reporting.
Seamlessly Integrate with Existing Systems
Connect LogisticsOS with pharmaceutical ERP platforms, warehouse management systems (WMS), and regulatory tracking tools through a robust API—ensuring seamless integration with existing workflows while maintaining compliance with Good Distribution Practices (GDP).
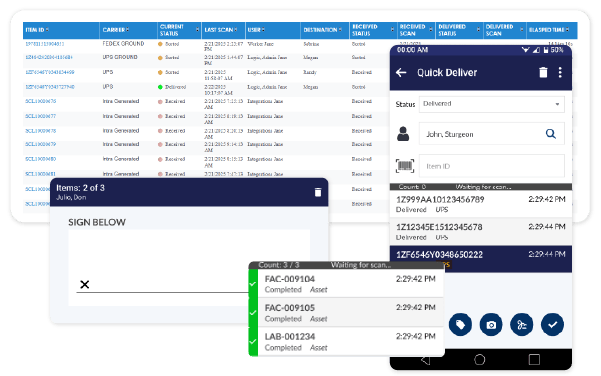
Achieve Real-Time Visibility
Gain complete transparency with real-time tracking of active pharmaceutical ingredients (APIs), clinical trial samples, and temperature-sensitive biologics. Secure audit trails provide instant access to shipment status, reducing risk and ensuring regulatory compliance.
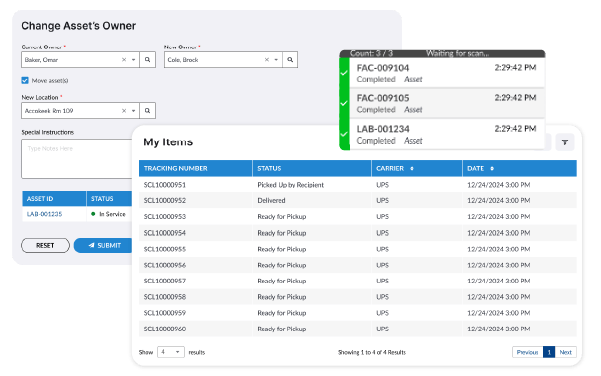
Ensure Compliance with Ease
Maintain full compliance with pharmaceutical regulations by automating audit records, securing digital proof of delivery, and ensuring proper cold-chain monitoring. LogisticsOS provides the tools necessary for pharmaceutical companies to meet strict Good Manufacturing Practices (GMP) and FDA/EMA guidelines.
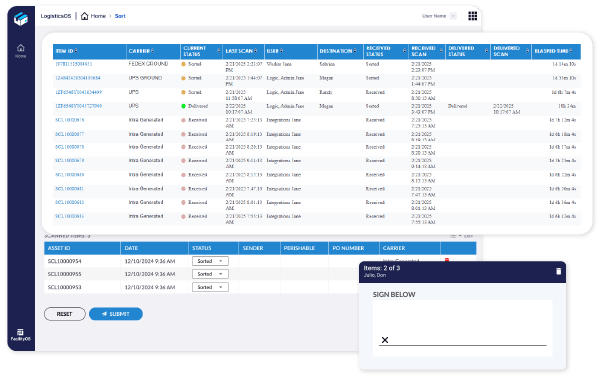
Drive Operational Efficiency
Eliminate bottlenecks and reduce manual errors with automated inventory, package, and asset tracking. LogisticsOS adapts to both high-volume distribution and specialized handling needs, ensuring efficiency at every step of the pharmaceutical supply chain.
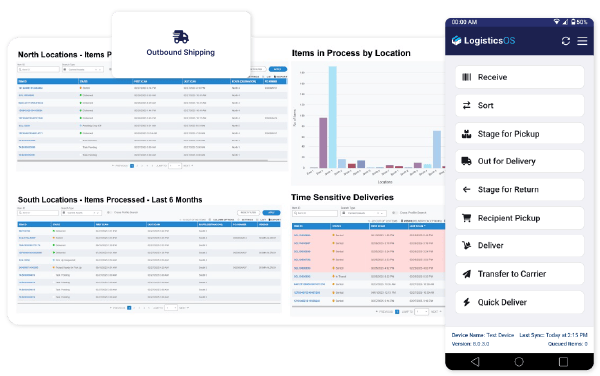
Scale with Confidence
Support multi-location pharmaceutical manufacturing and distribution with a centralized logistics platform. LogisticsOS ensures consistency, efficiency, and security as operations expand across international supply chains.
Pharmaceutical Industry Compliance Requirements
Pharmaceutical manufacturers, distributors, and facilities must comply with strict regulations to ensure drug safety, traceability, and proper handling. A robust logistics management system helps organizations stay compliant by providing real-time tracking, secure documentation, and automated audit trails.
U.S. Food and Drug Administration (FDA) – Drug Supply Chain Security Act (DSCSA)
The DSCSA requires pharmaceutical manufacturers, wholesalers, and dispensers to maintain a secure chain of custody and detailed tracking of prescription drugs throughout the supply chain.
LogisticsOS helps ensure compliance by:
- Tracking pharmaceutical shipments in real-time to prevent counterfeiting and diversion.
- Maintaining digital proof-of-delivery and audit logs for compliance reporting.
- Providing automated lot tracking to support recalls and traceability efforts.

World Health Organization (WHO) – Good Manufacturing Practices (GMP)
The WHO's GMP guidelines require pharmaceutical manufacturers to maintain rigorous quality control, documentation, and traceability of raw materials and finished products.
LogisticsOS helps ensure compliance by:
- Tracking raw materials and production batches through automated chain-of-custody.
- Maintaining audit trails for quality assurance and compliance validation.
- Providing real-time alerts for inventory discrepancies or compliance risks.

European Medicines Agency (EMA) – Good Distribution Practice (GDP) for Pharmaceuticals
The EMA's GDP guidelines ensure the quality of medicines during distribution, requiring strict temperature control, documentation, and security measures.
LogisticsOS helps ensure compliance by:
- Ensuring secure chain-of-custody tracking for medical shipments.
- Automating storage temperature monitoring for perishable medications.
- Providing compliance-ready digital reports for regulatory audits.
Health Canada – Good Manufacturing Practices (GMP) for Pharmaceutical Logistics
Health Canada’s Good Manufacturing Practices (GMP) set strict requirements for the manufacturing, storage, and distribution of pharmaceuticals, ensuring product integrity and regulatory compliance. These principles also apply to pharmaceutical shipments, including mail, parcel, and asset logistics.
LogisticsOS helps ensure compliance by:
- Ensuring traceability with end-to-end tracking of pharmaceutical shipments, providing visibility from origin to destination.
- Real-time shipment monitoring to maintain temperature control for cold chain logistics, preventing spoilage and ensuring product efficacy.
- Automating compliance documentation by capturing delivery details and proof of receipt, streamlining record-keeping for regulatory audits.

Who Benefits from a Logistics Management System?
Pharmaceutical Supply Chain & Logistics Managers
Gain full visibility into drug shipments, supplier performance, and distribution efficiency. Reduce shipment delays, prevent counterfeit medications, and optimize warehouse operations with automated tracking and reporting.
Cold Chain & Quality Control Managers
Ensure compliance with Good Distribution Practice (GDP) by tracking temperature-sensitive shipments, automating documentation, and preventing excursions in pharmaceutical storage and transportation.
Procurement & Receiving Officers Icon
Streamline supplier deliveries and raw material management with automated workflows. Verify, sort, and track shipments in real time while maintaining compliance-ready procurement records.
Compliance & Audit Teams
Eliminate manual reporting inefficiencies with automated compliance tracking. LogisticsOS provides complete chain-of-custody transparency and audit-ready documentation to support global pharmaceutical regulations.
IT & Security Administrators
Ensure secure integration with pharmaceutical ERP and compliance systems, manage access control for logistics operations, and maintain compliance with cybersecurity and data protection regulations.
What Can LogisticsOS Do for You?
Request a demo to see how LogisticsOS can deliver immediate results for your organization.
Book a Demo
This webpage and its content are an interpretation of the compliance requirements and is not legal advice nor should it act as a replacement for having a legal team review the specific compliance needs of your organization.