Emergency & Evacuation Management for the Pharmaceutical Industry
Improving Emergency Response for Pharma Facilities

Why Pharmaceutical Facilities Need Emergency Management
Pharmaceutical facilities operate under strict regulatory standards set by organizations like the FDA and Health Canada. These standards require robust emergency preparedness and evacuation management to ensure employee safety, protect intellectual property, and maintain compliance. EmergencyOS streamlines these processes, enabling efficient emergency responses and audit-ready documentation, which is essential for maintaining a safe and productive environment.
How Can EmergencyOS Help Pharmaceutical Facilities?
Digitize Evacuation Processes
Say goodbye to inefficient manual evacuation processes. EmergencyOS digitizes and automates evacuation procedures, eliminating error-prone paper-based methods. This reduces costly downtime and ensures compliance with Good Manufacturing Practices (GMP) by maintaining accurate digital records.

Extend Communication
Remove communication barriers for non-employees, including contractors and temporary visitors. Integration with Visitor Management Systems, such as VisitorOS, ensures that all personnel are included in real-time emergency communication channels, ensuring safety and compliance across your facility.

Communicate in Real-Time
EmergencyOS enables Safety Officers to communicate instantly with all evacuees via SMS and email, ensuring timely updates and instructions during emergencies. This feature is critical for pharmaceutical facilities where timely communication can protect sensitive operations and intellectual property.

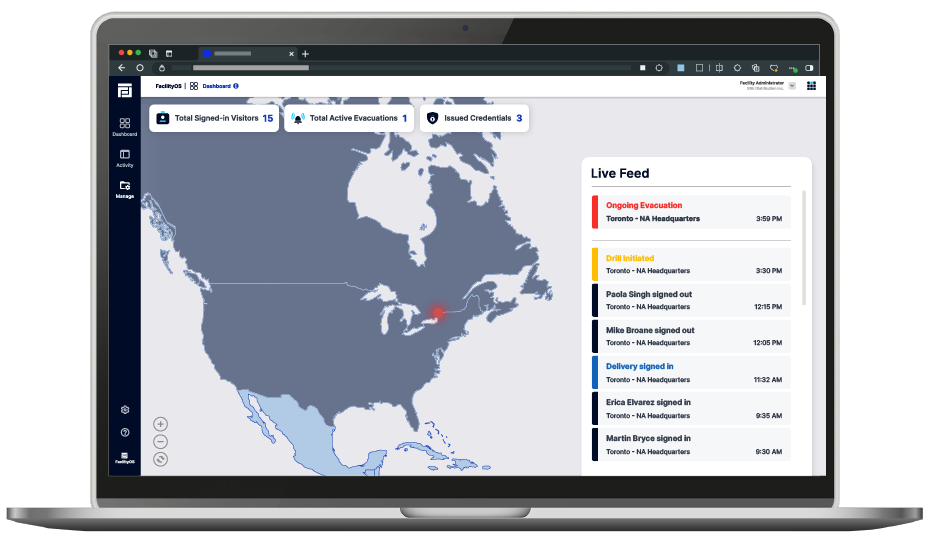
Gain Real-Time Visibility
Get up-to-the-minute visibility into the status of employees, contractors, and visitors during an emergency. EmergencyOS provides a centralized interface, ensuring that Safety Officers and Compliance Managers have oversight over protecting personnel and sensitive materials.

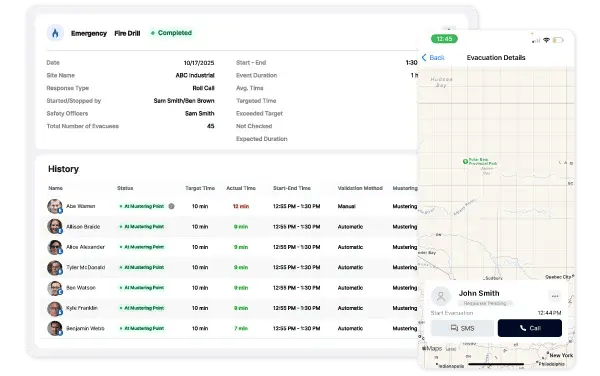
Perform Digital Roll Calls
Manual paper-based roll calls are time-consuming and often incomplete. With EmergencyOS, Safety Officers can save valuable time with digital roll calls. The latest status from all evacuees is automatically gathered. Have multiple mustering points? No problem! The data is stored in a remotely accessible central location.

Tailor Emergency Responses
Collect and store evacuation data such as the nature of the event, emergency event duration, individual evacuation times, and more. EmergencyOS provides a digital record of evacuation events along with complied detailed evacuation metrics for all facilities providing company-wide visibility across your entire enterprise. This ensures compliance with FDA and Health Canada requirements by providing comprehensive audit trails and evacuation metrics across all facilities.

Evacuation Reporting
EmergencyOS automatically logs evacuation events, capturing details like response times, incident duration, and personnel movements to help organizations analyze and refine emergency procedures over time.

Compliance Reporting
EmergencyOS maintains detailed digital reports essential for internal and external audits. It simplifies compliance with stringent pharmaceutical regulations by ensuring accurate record-keeping, reducing the risk of penalties and costly audits.

Data Driven Improvements
Leverage historical evacuation data to track trends, identify friction points, and enhance emergency response strategies. EmergencyOS empowers pharmaceutical facilities to make data-driven improvements, ensuring continuous operational safety and compliance.
Pharmaceutical Compliance Requirements
Pharmaceutical facilities must comply with strict regulatory standards to ensure safety, quality, and operational continuity. EmergencyOS helps simplify compliance with real-time tracking, automated reporting, and digitized emergency workflows—ensuring facilities meet the requirements of various safety regulations.
Occupational Safety and Health Administration (OSHA)
OSHA mandates strict safety and emergency preparedness requirements to protect employees from workplace hazards, including chemical exposure, fires, and other emergencies in pharmaceutical facilities. Compliance involves having well-documented evacuation plans, safety drills, and real-time communication during emergencies. Failing to comply can result in significant fines and legal consequences.
EmergencyOS enables compliance by:
- Digitizing evacuation procedures and mustering processes.
- Enhancing communication with real-time alerts and status updates.
- Providing detailed compliance reports for audits and inspections.

Good Manufacturing Practices (GMP)
GMP regulations ensure that pharmaceutical products are produced consistently and controlled according to strict quality standards. This includes rigorous requirements for safety, emergency preparedness, and evacuation protocols to protect product integrity, personnel safety, and facility security. Non-compliance can lead to costly recalls, regulatory penalties, and reputational damage. An emergency management system, like EmergencyOS, streamlines emergency management, ensuring regulatory adherence and operational continuity.
EmergencyOS supports compliance by:
- Providing digital records of emergency drills and evacuation events for GMP audits.
- Ensuring real-time communication and tracking of all personnel during emergencies.
- Offering automated emergency response procedures aligned with GMP safety protocols.

Food and Drug Administration (FDA)
The FDA enforces Good Manufacturing Practices (GMP) and facility security standards to ensure pharmaceutical products are consistently produced under safe and controlled conditions. This includes stringent requirements for emergency preparedness, evacuation procedures, and personnel safety to minimize risks of contamination, product recalls, and operational disruptions. Non-compliance can lead to severe penalties, operational shutdowns, and legal liabilities. An emergency management system, like EmergencyOS, helps facilities efficiently manage emergency responses while maintaining compliance with FDA standards.
EmergencyOS enables compliance by:
- Maintaining digital audit trails of all evacuation events.
- Ensuring accurate headcounts and real-time status updates.
- Providing customizable emergency response procedures tailored to GMP requirements.

Health Canada
Health Canada regulates pharmaceutical manufacturing to ensure compliance with Good Manufacturing Practices (GMP) and safety standards. This includes maintaining secure and safe working environments with robust emergency preparedness and evacuation protocols. Non-compliance can result in product recalls, operational shutdowns, and legal repercussions. An emergency management system, like EmergencyOS, helps pharmaceutical facilities manage emergency responses while maintaining Health Canada’s stringent safety requirements.
EmergencyOS supports compliance by:
- Maintaining detailed evacuation logs and audit trails.
- Facilitating real-time communication with all personnel during emergencies.
- Customizing emergency responses to meet Health Canada's safety standards.

ISO 13485 (Quality Management systems in Medical Devices and Pharmaceuticals)
ISO 13485 is an international standard for quality management systems in medical devices and pharmaceuticals, requiring organizations to maintain robust safety and emergency response procedures. Compliance involves maintaining accurate records of safety drills, evacuation events, and personnel safety measures. Non-compliance can lead to legal liabilities and product recalls. EmergencyOS supports facilities in maintaining ISO 13485 standards by ensuring accurate documentation and real-time communication during emergencies.
EmergencyOS enables compliance by:
- Providing digital records of emergency drills and evacuation events.
- Ensuring accurate compliance reporting for quality management audits.
- Enabling data-driven improvements for continuous safety enhancements.

ISO 45001 (Occupational Health and Safety Management Systems)
ISO 45001 specifies requirements for occupational health and safety management systems to minimize workplace risks and enhance safety protocols. It includes strict guidelines for emergency preparedness, evacuation procedures, and safety reporting. Non-compliance can lead to accidents, legal liabilities, and operational disruptions. EmergencyOS supports compliance by providing centralized oversight and real-time visibility into safety procedures, through:
- Offering real-time visibility into employee safety during emergencies.
- Automating evacuation processes for improved safety management.
- Providing detailed compliance reports for ISO audits and certifications.

General Data Protection Regulation (GDPR)
GDPR mandates strict data privacy and protection requirements when handling visitor and employee information in pharmaceutical facilities, especially those operating globally. Non-compliance can lead to substantial fines and legal penalties. An emergency management system, like EmergencyOS, ensures data security and privacy during emergency management by safeguarding personnel information and maintaining digital audit trails.
EmergencyOS enables compliance by:
- Ensuring secure storage and management of evacuation and personnel data.
- Providing complete digital audit trails for data protection audits.
- Allowing configurable data retention policies for regulatory compliance.

Who Benefits from an Emergency Management System?
Security & Safety Officers
Ensuring employee safety while maintaining regulatory compliance is critical for Safety Officers. EmergencyOS facilitates instant communication, digital roll calls for fast headcounts, and customizable emergency responses tailored to GMP and OSHA requirements.
Facility Managers
Facility Managers in pharmaceutical facilities manage critical infrastructure and regulatory compliance. EmergencyOS streamlines evacuation procedures, centralizes oversight, and maintains adherence to GMP and FDA safety standards, ensuring operational continuity.
Visitors & Contractors
Pharmaceutical facilities frequently host visitors, including regulatory inspectors, contractors, and suppliers. EmergencyOS connected with a visitor management system, like VisitorOS, includes them into emergency communication channels, ensuring real-time alerts, digital mustering, and compliance with visitor safety protocols.
Security Managers
Security Managers safeguard personnel and sensitive areas during emergencies. EmergencyOS provides real-time visibility, coordinated evacuation management, and efficient communication, ensuring safety and compliance with industry regulations.

Intuitive, easy to use
We rolled out 90 locations quickly in about 3 months with the help of iLobby's support team.
Jeff O.
Field Service Leader


iLobby is EXCELLENT for contractor management!!
even used the iLobby system to warn us if contractors had not left the site after a certain period of time, but typically should have.
Dee M.
Health Safety Environment Coordinator


I highly recommend iLobby
Knowing who is at your facility and having the ability to alert them to evacuate if necessary is exceptionally beneficial.
Todd C.
EHS Manager


iLobby runs itself
instance viewing of everyone coming and going plus add visitors in advance.
Robert I.
IT Automation Technician


Ton of features
Solved our problem of maintaining annual paperwork for rules and regulations for visitors and contractors.
Nicole F.
Food Safety and Quality Assurance Manager


“Great Experience”
A lot more robust & easier to navigate than paper. Emergency notices are also a big feature that we use in the case of emergencies at our sites to communicate to our employees.
Travis E.
IT Plant Support Analyst, Infrastructure & Security


“Extremely Versatile”
This software allows us to notify our employees in case of emergency all with one simple set up per employee.
Jennifer A.
Manager, General Services Lactalis USA


"iLobby is EXCELLENT for contractor / visitor management!!"
The iLobby system is easy to use, the software is user friendly, and there are multiple ways to take advantage of the capabilities of the system.
Dee M.
Health Safety Environment Coordinator


"Ease of implementation and use."
Visitors have been impressed with how easy and advanced our Check-in process is.
Chad J.
Director of Supply Chain


"Definitely recommend"
We have experienced zero issues with visitors checking in which is all you can ask for with a visitor mgmt platform!
Molly V.
Executive Assistant, Operations


"Easy set up, easy to use"
Its easy to use, very customizable and was very quick and simple to implement.
Billy L.
Building Automation Systems Manager


"A very user friendly product!
We had a very easy time implementing this as our visitor management solution.
Usama M.
IT Supervisor, Plant Support

What Can EmergencyOS Do for You?
Request a demo to see how EmergencyOS can deliver immediate results for your organization.
Book a Demo
This webpage and its content are an interpretation of the compliance requirements and is not legal advice nor should it act as a replacement for having a legal team review the specific compliance needs of your organization.