PIAM for Pharmaceutical Enterprises
Extend the Benefits of Access Control to Visitors & Contractors
Why Pharmaceutical Companies Need PIAM & Access Control Management
The pharmaceutical industry operates under tight security protocols due to the high value of its intellectual property, strict regulatory standards, and the need to maintain product integrity. SecurityOS is essential for pharmaceutical companies to safeguard their facilities, manage physical access to critical areas, and ensure compliance with ever-evolving industry regulations. The automated approach of SecurityOS helps companies streamline their security efforts and maintain operational continuity in an increasingly complex landscape.
How Can SecurityOS Help Pharmaceutical Companies?

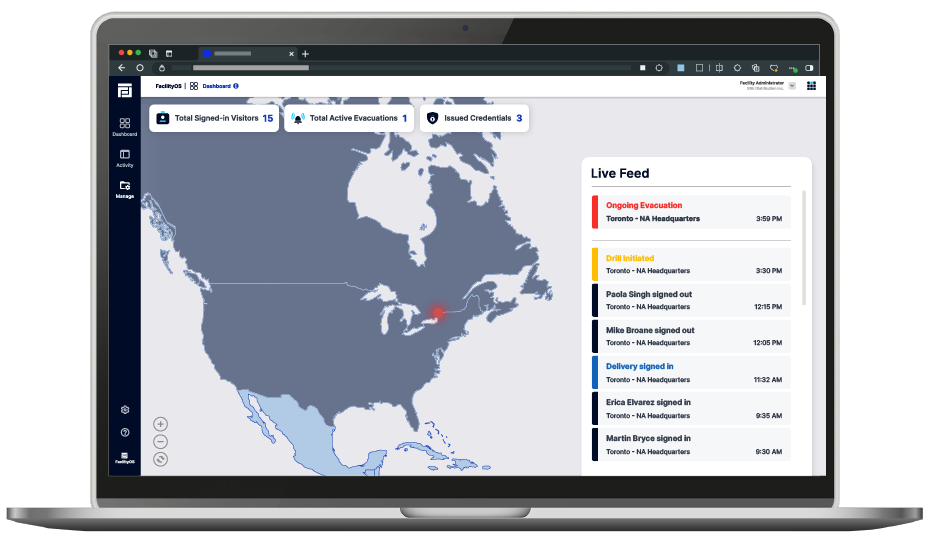
Centralized Access Control
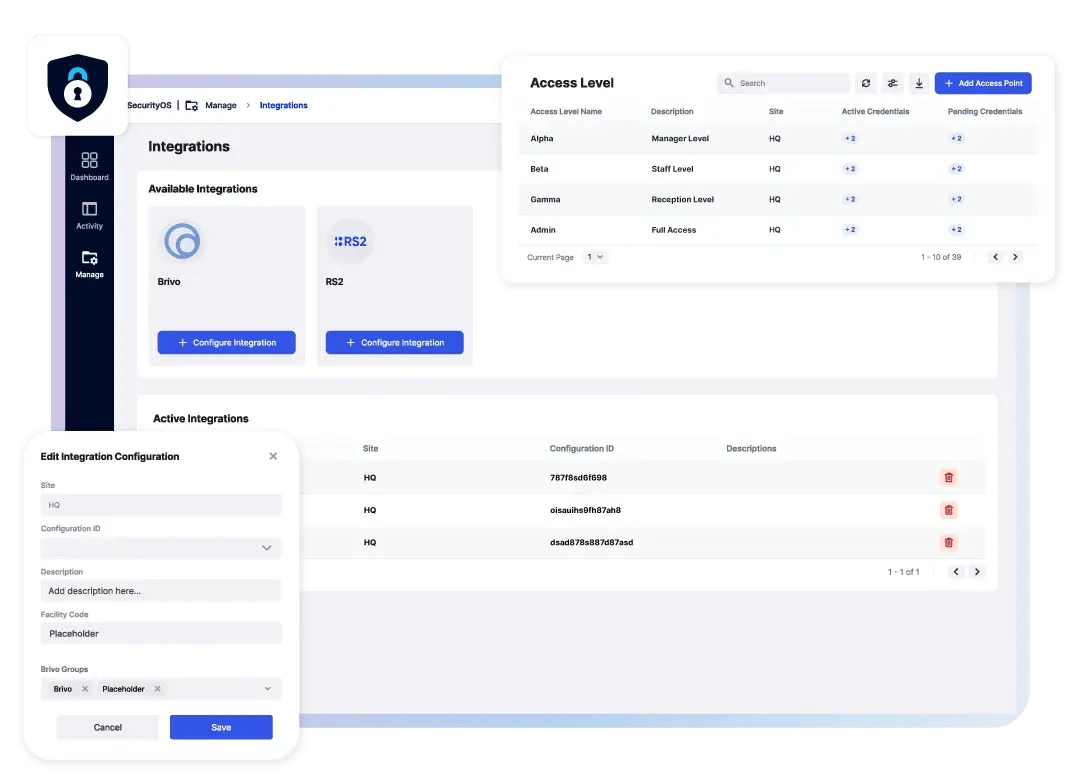
Easily manage access to pharmaceutical facilities, labs, production areas, and research zones. With SecurityOS, you can set customized permissions to control access to sensitive zones such as R&D labs, production lines, and secure storage areas, based on employee roles, work shifts, and project timelines. This helps ensure that only authorized guests are granted physical access.

Automate Provisioning
Automate the process of issuing and revoking access credentials for temporary workers, contractors, and visitors. SecurityOS streamlines the access process, reducing human error and ensuring that only approved personnel can enter high-security areas like clean rooms, chemical storage, and laboratories. By automating this process, SecurityOS enhances security and operational efficiency.

Compliance & Reporting
Ensure compliance with stringent regulatory requirements like FDA and GMP with detailed audit logs and reports. SecurityOS tracks access to critical areas, offering visibility into who accessed sensitive pharmaceutical facilities, what areas they entered, and when. These comprehensive records are invaluable for security audits, regulatory inspections, and compliance reviews.

Expire on Sign-Out
For temporary workers, contractors, and visitors, SecurityOS helps ensure that access is automatically revoked when it's no longer needed. After a specific time or upon sign-out, access credentials expire, minimizing the risk of unauthorized re-entry and ensuring that only authorized individuals remain in sensitive areas, such as during off-hours or after shifts.

Centralized Management
Managing access across multiple pharmaceutical sites is made easy with SecurityOS. The centralized platform enables administrators to oversee and control access to all facilities from a single interface, ensuring that security policies are consistently enforced and operational efficiency is maintained across all sites.

Control Points of Entry
Whether using FacilityOS hardware or integrating with existing access control systems, SecurityOS helps ensure secure control of key entry points to pharmaceutical facilities. It enhances your access control system, providing the flexibility to manage complex security needs across various areas, including labs, production lines, and secure storage zones.

Data Privacy and Compliance
With the pharmaceutical industry facing strict data privacy regulations, SecurityOS helps ensure compliance by protecting personal information collected during access management. Sensitive data is securely handled in accordance with data privacy laws, protecting personnel and patient information while maintaining compliance with regulations like GDPR.
Pharmaceutical Compliance Requirements
The pharmaceutical industry is heavily regulated to ensure that products are safe, effective, and manufactured under strict conditions. Compliance with these regulations is critical to protecting public health, safeguarding intellectual property, and avoiding legal consequences. SecurityOS helps pharmaceutical companies meet these regulatory requirements, enhance security, and reduce operational risks.
Occupational Safety and Health Administration (OSHA)
OSHA regulates workplace safety, ensuring that pharmaceutical workers and visitors are protected from potential hazards, including exposure to biohazards or infectious diseases. SecurityOS helps ensure compliance by:
- Restricting access to high-risk areas, such as labs handling hazardous materials.
- Tracking the entry and exit of personnel to safety-critical areas and helping ensure that only qualified and trained individuals can enter those zones.
- Providing detailed audit trails to support OSHA safety audits and incident investigations.

Good Manufacturing Practices (GMP)
Good Manufacturing Practices (GMP) are essential for ensuring that pharmaceutical products are consistently produced under controlled conditions. Compliance with GMP is vital for maintaining product quality and safety. SecurityOS supports GMP compliance by:
- Controlling and limiting access to manufacturing areas where sensitive pharmaceutical products are produced or tested.
- Ensuring that only trained, qualified personnel can access production lines, laboratories, and clean rooms.
- Providing audit trails of who accessed GMP-regulated zones, supporting audits and ensuring regulatory adherence.

Food and Drug Administration (FDA) Regulations
The FDA enforces regulations to ensure the safety and efficacy of pharmaceutical products. Compliance with FDA guidelines is critical for pharmaceutical manufacturers. SecurityOS helps with FDA compliance by:
- Managing access to areas where controlled substances, chemicals, and pharmaceuticals are stored and handled.
- Providing detailed logs of who accessed sensitive areas and when, helping to demonstrate compliance during FDA inspections.
- Automating access provisioning for temporary workers and contractors, ensuring they are only allowed in authorized zones.

Who Benefits from a PIAM & Access Control Management System?
Quality Assurance & Compliance Managers
Quality Assurance & Compliance Managers are responsible for ensuring pharmaceutical facilities meet strict regulatory requirements such as FDA guidelines. SecurityOS helps them track and control access to critical areas like clean rooms and laboratories, maintain detailed audit trails, and automate compliance reporting. This allows them to streamline regulatory inspections and minimize the risk of violations.
Facility Operations Managers
Facility Operations Managers are responsible for maintaining the security and efficiency of pharmaceutical production sites. SecurityOS provides them with a centralized platform to oversee access permissions, enforce security policies, and integrate with existing access control systems. By automating access expiration for temporary staff and tracking facility entry points, they can optimize security while ensuring seamless operational efficiency.
Pharmaceutical Security Directors
Pharmaceutical Security Directors oversee access control and security measures to protect intellectual property, secure research labs, and prevent unauthorized entry into sensitive production zones. SecurityOS enables them to centrally manage access across multiple sites, automate provisioning for temporary workers, and ensure compliance with data privacy laws. By leveraging automated security protocols, they can mitigate risks and enhance facility protection.

Intuitive, easy to use
We rolled out 90 locations quickly in about 3 months with the help of iLobby's support team.
Jeff O.
Field Service Leader


iLobby is EXCELLENT for contractor management!!
even used the iLobby system to warn us if contractors had not left the site after a certain period of time, but typically should have.
Dee M.
Health Safety Environment Coordinator


I highly recommend iLobby
Knowing who is at your facility and having the ability to alert them to evacuate if necessary is exceptionally beneficial.
Todd C.
EHS Manager


iLobby runs itself
instance viewing of everyone coming and going plus add visitors in advance.
Robert I.
IT Automation Technician


Ton of features
Solved our problem of maintaining annual paperwork for rules and regulations for visitors and contractors.
Nicole F.
Food Safety and Quality Assurance Manager


“Great Experience”
A lot more robust & easier to navigate than paper. Emergency notices are also a big feature that we use in the case of emergencies at our sites to communicate to our employees.
Travis E.
IT Plant Support Analyst, Infrastructure & Security


“Extremely Versatile”
This software allows us to notify our employees in case of emergency all with one simple set up per employee.
Jennifer A.
Manager, General Services Lactalis USA


"iLobby is EXCELLENT for contractor / visitor management!!"
The iLobby system is easy to use, the software is user friendly, and there are multiple ways to take advantage of the capabilities of the system.
Dee M.
Health Safety Environment Coordinator


"Ease of implementation and use."
Visitors have been impressed with how easy and advanced our Check-in process is.
Chad J.
Director of Supply Chain


"Definitely recommend"
We have experienced zero issues with visitors checking in which is all you can ask for with a visitor mgmt platform!
Molly V.
Executive Assistant, Operations


"Easy set up, easy to use"
Its easy to use, very customizable and was very quick and simple to implement.
Billy L.
Building Automation Systems Manager


"A very user friendly product!
We had a very easy time implementing this as our visitor management solution.
Usama M.
IT Supervisor, Plant Support

What Can SecurityOS Do for You?
Request a demo to see how SecurityOS can deliver immediate results for your organization.
Book a Demo
FacilityOS understands that customer’s requirements are subject to regional regulatory framework concerning privacy and data protection. Thus, the compliance requirements below will depend on specific configuration of FacilityOS's product(s) that customer would want to incorporate into its processes or systems. Yet, to provide a general protection concerning privacy, FacilityOS's products support the right to be forgotten, and extensive data residency and data export requirements. Further, FacilityOS also functions as a data processor on behalf of its customer (controller) when it comes to data compliance connected to GDPR.