GMP or Good Manufacturing Practice, sets quality assurance standards for the production of food, pharmaceuticals, and medical devices. It ensures consistent manufacturing and control processes, focusing on documentation, personnel training, raw material control, equipment validation, and quality control measures. Adhering to GMP is crucial for product safety and effectiveness, emphasizing the need for a clean environment and regular testing. GMP compliance is essential for industries to meet defined quality standards globally.

How Can FacilityOS Help with GMP Compliance?
Ensuring compliance with Good Manufacturing Practice (GMP) standards is crucial for maintaining consistent quality in manufactured goods. Manufacturers must track on-premises activities and enforce standard operating procedures (SOPs). FacilityOS's facility and visitor management platform, FacilityOS, aids in meeting these requirements through robust data security, physical security, and access control processes.
FacilityOS offers visitor management functionalities that align seamlessly with GMP standards, especially in managing and documenting visitor and contractor compliance with GMP protocols.
Induction and Visitors Training
Ensure visitors receive necessary induction training on GMP practices and log their participation as part of the sign-in process.
Visitor Screening and Hygiene Compliance
Screen visitors for compliance with essential hygiene standards, ensuring they adhere to your facility's GMP protocols.
Audit and Inspection Readiness
Quickly provide comprehensive visitor data during audits and inspections, demonstrating adherence to GMP regulations.
Restrict Access
Manage and restrict visitor access to sensitive production areas to prevent contamination, a key requirement under GMP.
Audit Trails
Maintain comprehensive digital logs of all visitor activity, including areas accessed, to meet GMP’s stringent record-keeping requirements.
Incident Reporting and Response
Facilitate prompt incident reporting and response mechanisms, an important aspect of GMP compliance.
Emergency Response Coordination
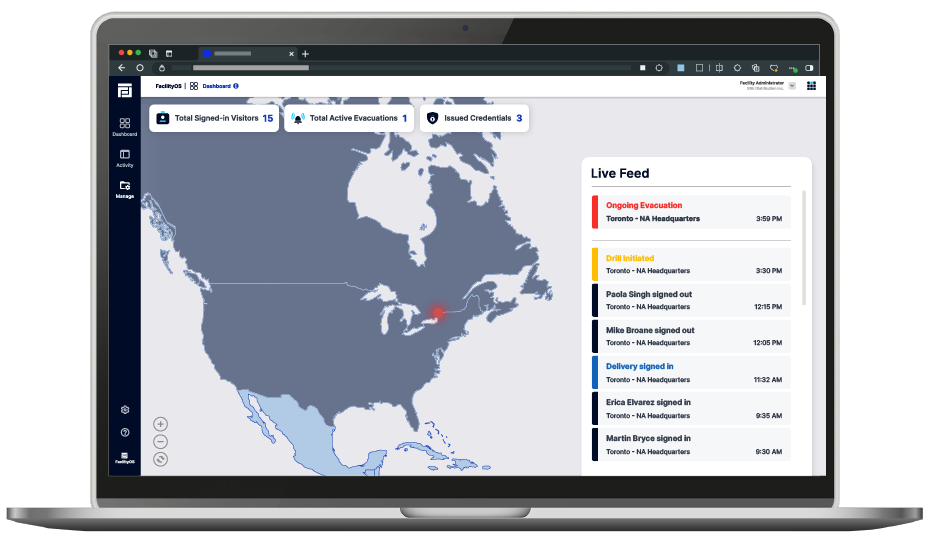
Utilize real-time visitor information for effective emergency and evacuation management, a vital component of GMP compliance in ensuring safety.
GMP Agreement Signatures
Capture digital signatures on GMP agreements from visitors and contractors, ensuring they understand and commit to your facility's GMP protocols within your manufacturing environment.
Data Protection
We stay current with industry standards, enforce rigorous data security measures, and undergo regular audits to verify adherence to established information security processes and systems, ensuring the protection of sensitive information.
Enhance GMP Compliance through Effective Visitor Management
Request a demo to see how FacilityOS can assist your organization in effectively meeting GMP requirements. Book a Demo
This webpage and its content are an interpretation of GMP requirements and is not legal advice nor should it act as a replacement for having a legal team review the specific compliance needs of your organization.